High arterial blood pressure (A.K.A. hypertension) attacks over 1/3 of adult population worldwide. As a “silent killer”, hypertension exacerbates lethal outcome of cardiovascular diseases such as heart attack, heart failure, stroke and kidney failure. Despite its etiological obscurity, a pronounced elevation of sympathetic nerve activity (SNA) has been well recognized in propelling hypertension progression. Renal sympathetic denervation has been successfully used to treat resistant hypertension patients in clinic, underscoring the pivotal role of sympathoexcitation in hypertension. However, the ultimate question, i.e., how SNA is unleashed during hypertension remains unresolved.
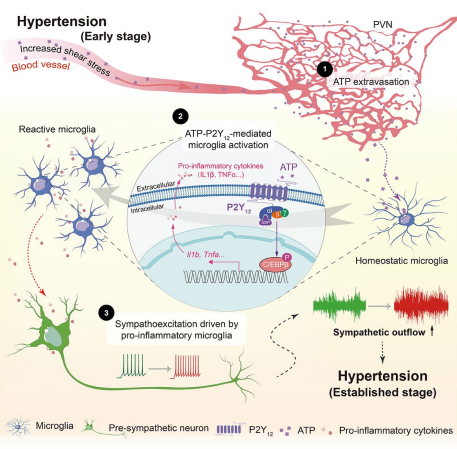
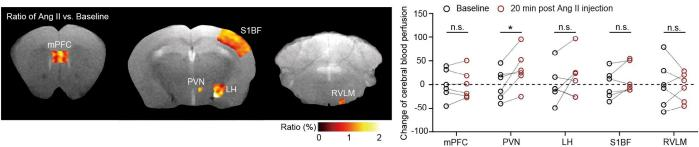
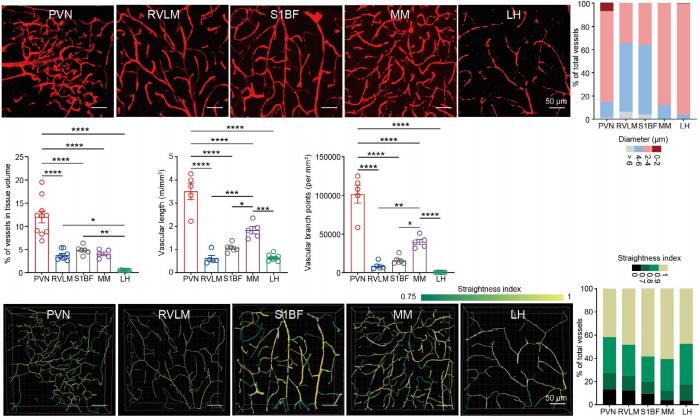
On August 7, 2024, Immunity published a study entitled “Microglia in the hypothalamic paraventricular nucleus sense hemodynamic disturbance and promote sympathetic excitation in hypertension” by Dr. SHI Peng from Zhejiang University Institute of Translational Medicine. In this study, it shows that a subtle but significant increase of plasma ATP triggers microglia, the resident immune cells in the brain, switching from a surveillant towards a reactive state. ATP is an intracellular “cash” to supply energy for biochemical reactions. An increase of extracellular ATP is considered as a danger signal to immune cells. Nearly all immune cells express various purinergic receptors, which render the “police cells” remarkably alert to cell or tissue damages. When blood cells such as red blood cells experience increased sheer stress due to blood pressure increase, intracellular ATP would be released via transiently opened pannexin channels, leading to an ATP elevation in blood plasma. This blood-borne ATP is detected by purinergic receptors P2Y12 of microglia. By using a series of loss-of-function approaches, the researchers finally validated that ATP-P2Y12 ligation is required for microglial activation, which subsequently promotes the electronic activity of sympathetic neurons by secreting proinflammatory mediators. Consequentially, it leads to a massive and sustained elevation in SNA and blood pressure. More importantly, this study unravels a precedently unappreciated microanatomic structure in the brain which predisposes the paraventricular nucleus (PVN), a hub of sympathetic regulation, susceptible to hemodynamic alteration. The research group found that the PVN possesses the highest microvessel density, the thinnest lumen diameter, the least straightness, and the most ramification compared with the other examined brain regions (Figure 1). As such, the cerebral blood flow would be slower and leave sufficient time window for small molecules, e.g., ATP (507 Da) to undergo extravasation. With the aid of magnetic resonance imaging (MRI), this study found that transient increase of arterial blood pressure resulted in an exclusive increase of blood perfusion in the PVN rather than other brain regions (Figure 2). Altogether, the data strongly indicate that a unique vascular topology renders the PVN receiving more blood-borne ATP in blood pressure increase, which drives a sympathetic overflow in hypertension.
Blood pressure is a vital factor warranting organs and tissues to receive sufficient blood perfusion, which is tightly regulated by a series of neuronal circuitry including evolutionally conserved negative feedback. Vasoconstriction of arteries or arterioles caused by persistently increased SNA is highly associated with hypertension. How SNA is raised despite the existing negative brakes in hypertension is a long-lasting mystery. Current study provides a novel answer to that. Local microglia would sense blood chemicals and initiate a cross-talk with neurons, which relies on a featured vasculature topology in the PVN. Breaking central ATP-P2Y12 signal pathway could be a potential therapeutical intervention for hypertension and other relevant diseases.