On 28 Augest, the research team led by Dr. SUN Jihong from the Department of Radiology, Sir Run Run Shaw Hospital of Zhejiang University School of Medicine in cooperation with Prof. LIU Xiangrui from College of Chemical and Biological Engineering of Zhejiang University, and Prof. Zhen Cheng fromStanford University Medical Centerdeveloped a novel strategy combining chemotherapy and immunotherapy to modulate the tumor microenvironment (TME) using triblock copolymer nanoparticles, which enhances antigen cross-presentation and induces the conversion of the immunosuppressive TME to immunogenic TME.The findings are published in a research article titled “Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity” in Science Advances.
Cancer immunotherapy has led to unprecedented success in treating a diversity of cancers and improving survival. Nevertheless, only a minority of patients with given cancer types benefit from the present immunotherapies [for example, immune checkpoint blockers (ICBs)]. In particular, the patient’s response to immunotherapy and survival rate is closely correlated with the TME. The tumors with immunogenic “hot” TME, highly infiltrated with CD8+ T cells, exhibit the best response to ICB immunotherapy. In contrast, patients with “cold” tumors — so-called immune deserts, uninfiltrated with CD8+ T cells — do not benefit from ICB immunotherapy, highlighting the urgent need to identify new approaches to convert cold tumors into responsive hot tumors.
Growing evidence indicates that chemotherapy is not only tumor eliminative but also a positive modulator of the immune system through different mechanisms, such as modulating the TME, motivating scientists to launch clinical trials of chemotherapy-immunotherapy combinations for patients with different cancers. Recently, the U.S. Food and Drug Administration approved chemotherapy-immunotherapy combinations for the treatments of metastatic non–small cell lung cancer (1) and programmed death-ligand 1 (PD-L1)–positive metastatic triple-negative breast cancer. However, the rationality, efficacy, and safety associated with the combinational regimes are still the major challenges limiting the broader implementation of the chemo-immunotherapy approaches (3, 4).
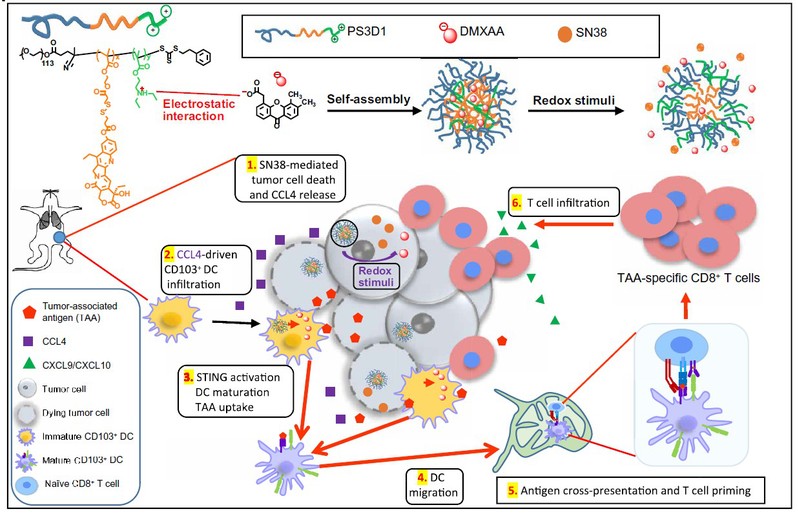 Sun Jihong et al. developed an anticancer chemo-immunotherapy approach achieved by novel nanoparticles systemically and co-delivering the chemotherapeutic agent SN38 (7-ethyl-10-hydroxycamptothecin) and the STING agonist DMXAA (5,6-dimethylxanthenone-4-acetic acid) that can convert the immunologically cold tumors to immunogenic hot tumors, yielding an enhanced expansion and tumor infiltration of tumor associated antigen (TAA)-specific CD8+ T cells that can potentiate strong antitumor immunity. The engineered nanosystem offers a rational design of an effective immunotherapy combination regimen, which is one of the top challenges in cancer immunotherapy.
Sun Jihong et al. developed an anticancer chemo-immunotherapy approach achieved by novel nanoparticles systemically and co-delivering the chemotherapeutic agent SN38 (7-ethyl-10-hydroxycamptothecin) and the STING agonist DMXAA (5,6-dimethylxanthenone-4-acetic acid) that can convert the immunologically cold tumors to immunogenic hot tumors, yielding an enhanced expansion and tumor infiltration of tumor associated antigen (TAA)-specific CD8+ T cells that can potentiate strong antitumor immunity. The engineered nanosystem offers a rational design of an effective immunotherapy combination regimen, which is one of the top challenges in cancer immunotherapy.
Please refer to the link of this study:https://advances.sciencemag.org/content/6/35/eabc3646