The congenital immune response is the body’s first line of defense against exotic pathogens and stressors. This rapid non-specific response relies on the capability of pattern recognition receptors in recognizing pathogen-related molecular patterns and damage-related molecular patterns released by damaged cells in a quick manner.
The cytosolic pattern recognition receptors (PRRs) nucleotide oligomerization domain 1 (NOD1) and NOD2 play crucial roles in host defense and survival, primarily by conferring responsiveness to cytosolic bacterial peptidoglycans [γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) and muramyldipetide (MDP)] shed by bacteria during infection.
Recently, the research team led by Prof. Dante Neculai from the Zhejiang University School of Medicine has proved that nucleotide oligomerization domain-like receptors 1 and 2 (NOD1 and NOD2), two proteins responsible for detecting bacterial products, require lipid modifications for their recruitment to the cell membrane and function. The specific modification, palmitoylation at a cysteine thiol, is mediated by the enzyme ZDHHC5. This discovery builds a bridge between scientific principles and clinical issues in an effective way, thus holding great promise in diagnosis and treatment. This study is published in the October 24 issue of the journal Science.
NOD1/2 are two major recognition receptors for congenital immunity to inflammatory bowel diseases (IBD). As“sentinels”, they assume different responsibilities. Some work on the “city wall” while others function in the “city wall”.
Researchers have found that NOD1/2 not only function well within the “firewall”, but they also work effectively next to the “firewall”. Nevertheless, NOD1/2 lack recognizable membrane-targeting domains and appear to be incompatible with the membrane. How can they act as a strong defender? Scientists have long been seeking for the story behind it.
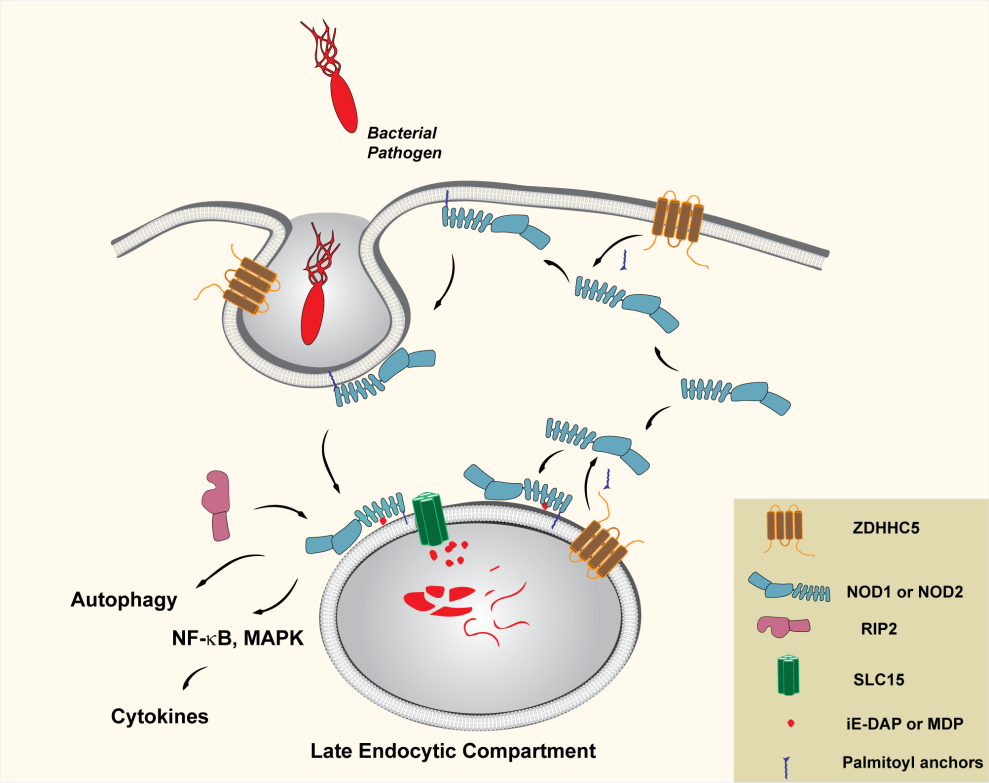
Dante Neculai et al. discover that NOD1/2 S-palmitoylation is indispensable for optimal membrane targeting and hence for proper immune signalingupon cognate peptidoglycan detection.Membrane association of NOD1/2is crucial for their function, as can be explicitly illustrated by the CD-associated NOD2 loss-of-function variant 3020 insC (3020 insC frame shift mutation that leads to a truncated NOD2 protein), which fails to associate with membranes and, as a consequence, is unable to detect bacterial intrusion. They provide evidence that NOD1/2 S-palmitoylation is vital not only for steady-state membrane association but also for ligand-induced signaling. Accordingly, S-palmitoylation–deficient mutants of NOD1/2 are mislocalized and lose their capacity to induce NF-kB signaling in response to C12-iE-DAP or MDP. Furthermore, they also find that ZDHHC5 is required for S-palmitoylation of NOD1/2, and both the presence of ZDHHC5 at the site of bacterial entry and its enzymatic activity are necessary for proper recruitment of NOD1/2 to the bacterial entrysite and phagosomes. Nevertheless, How ZDHHC5 is recruited to the site of bacterial entry remains an open question.

According to their research, a palmitoylation-dependent local accumulation of NODs, and their subsequent exposure to bacterial by-products, ensure a compartmentalized and efficient signaling response. Therefore, cells can respond to very low concentrations of bacterial products. Researchers propose that NOD1/2, together with ZDHHC5 and various transporters (such as SLC15A3), form specialized platforms for pathogen sensing. Their findings are expected to promote the understanding and ultimately the treatment of NOD-driven inflammatory diseases, including NOD-dependent autoimmune diseases and chronic bacterial infections.