The ability of animal cells to sense and adapt to oxygen availability is essential for life activities. William G. Kaelin Jr., Peter J. Ratcliffe and Gregg L. Semenza are awarded the 2019 Nobel Prize in Physiology or Medicine for their discoveries of how cells sense and adapt to oxygen availability. Mitochondria are the most crucial oxygen-consuming organelles in cells and are also the core sites of cell metabolism, providing sufficient energy and substances for cells. Due to the rapid growth of solid tumors, their core regions are often in a state of hypoxia. Under hypoxic conditions, mitochondria are vulnerable to damage, thus leading to cell apoptosis in tumor tissues. Key to tumor survival are the perception of the hypoxia microenvironment and the active balance of the contradiction between hypoxia and mitochondrial oxygen consumption.
LV Zhimin from the Zhejiang University Institute of Translational Medicine and QIAN Xu from the Nanjing Medical University School of Medicine engaged in collaborative research in the relevant field. Their findings are published in a research article entitled “KDM3A senses oxygen availability to regulate PGC-1a-mediated mitochondrial biogenesis” in the October 16 issue of the journal Molecular Cell.
Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1), includingPGC-1α, PGC-1b and PRC, plays a critical role in regulating multiple aspects of energy metabolism. Mitochondria biogenesis can be regulated through interactions with multiple transcriptionfactors, such as NRF 1/2, PPARa and ERRa. Researchers reveal that lysine demethylase 3A (KDM3A) binds to PGC-1α and demethylates monomethylated lysine (K) 224 of PGC-1α under normoxic conditions. Hypoxic stimulation inhibits KDM3A, which has a high KM of oxygen for its activity, and enhances PGC-1α K224 monomethylation. This modification decreases PGC-1α’s activity required for NRF1- and NRF2-dependent transcriptional regulation of TFAM, TFB1M, and TFB2M, resulting in reduced mitochondrial biogenesis. Expression of PGC-1α K224R mutant significantly increases mitochondrial biogenesis, reactive oxygen species (ROS) production, and tumor cell apoptosis under hypoxia and inhibits brain tumor growth in mice. This study suggests that PGC-1α monomethylation, which is dependent on oxygen availability-regulated KDM3A, plays a vital role in the regulation of mitochondrial biogenesis.
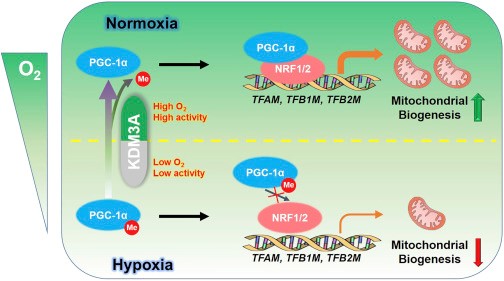
A Schematic Model Showing that Hypoxia-Induced PGC-1α K224 Monomethylation Suppresses Mitochondrial Biogenesis
This research offers a novel and essential mechanism underlying the hypoxia-induced inhibition of PGC-1α and the subsequent mitochondrial biogenesis by hindering KDM3A-mediated demethylation of monomethylated K224 of PGC-1α. Inhibition of PGC-1α K224 monomethylation immensely blocks brain tumor development, highlighting the potential to disrupt PGC-1a K224 regulation as an alternative approach to curing cancer.