Recently, the team of professor Zheng Shusen, the First Affiliated Hospital of Zhejiang University School of Medicine, published a research in the high-profile academic journal (HEPATOLOGY) (IF=14.09) entitled "Blocking TREM-1+ Tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-PD-L1 resistance in liver cancer". This article unveils that TREM-1 positive tumor-associated macrophages (TREM-1+ TAMs) induced by hypoxic microenvironment is the main cause of anti-PD-L1 therapy resistance, and blocking TREM-1+ TAMs provides possibility of reinvigorating efficient response to anti-PD-L1 treatment in hepatocellular carcinoma (HCC)

China has the highest incidence of HCC in the world. Although, surgical resection is recognized as a superior treatment for early stage patients, the majority of patients have lost valuable chances to operation because of the tumor progressed. Due to the limitations of the traditional targeted drugs Sorafenib and Regorafenib, the immune checkpoint drugs represented by the anti-PD-1/PD-L1 axis provide potential for patients with advanced HCC. Opdivo (Nivolumab), a drug targeting to the PD-L1/PD-1 axis, have been approved by FDA and entered China in June 2018 achieving favorable efficacy in various tumor treatments. However, the abundant of Tumor-infiltrating suppressive immunocytes in HCC imped the anti-tumor efficacy of PD-1/PD-L1 blockade, and the anti-PD-1/PD-L1 treatment is unresponsiveness. Preclinical studies demonstrated that the response rate of HCC against anti-PD-1/PD-L1 treatment was only 14.3%. Therefore, it is imperative to reverse the unresponsiveness of anti-PD-1/PD-L1 treatment, explore the novel therapeutic strategy, and improve patient prognosis.
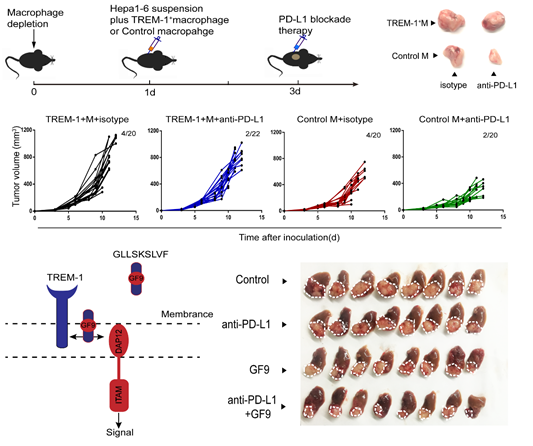
In this study, Zheng Shusen’s team unveils that the hypoxic microenvironment is the onset for the anti-PD-L1 resistance. Hypoxic microenvironment, a common feature of solid tumors, has the effects on drug resistance, angiogenesis, matrix remodeling, etc. However, how tumor-associated immune cells, especially tumor-associated macrophages (TAMs), response to the hypoxic context remains unclear. This article demonstrated that HIF-1α nuclear location significantly up-regulated the transcription level of TREM-1 by binding to the -936~-929 region upstream of the TREM-1 promoter. In addition, TREM-1+ TAMs were significantly related to the degree of tumor hypoxia, and the enrichment of TREM-1+ TAMs indicated tumor progression and poor prognosis. Predominately, it has elucidated that CCR6+ Treg recruited by TREM-1/ERK/NF-κβ/CCL20 signaling pathway was the main mechanism to endow HCC with the anti-PD-L1 resistance in the hypoxic microenvironment. Given that the specific ligand-independent TREM-1 inhibitor GF9 (GLLSKSLVF) abrogated the anti-PD-L1 therapeutic resistance and significantly improved anti-tumor efficacy by inhibiting the recruitment of CCR6+ Tregs, blocking TREM-1+ TAMs provides a theoretical basis and potential solution for the fact that most HCC patients cannot benefit from anti-PD-1/PD-L1 treatment.
Professor Zheng Shusen is the corresponding author of the article, and Wu Qinchuan is the first author. The above research was funded by key projects of the National Natural Science Foundation of China and innovative group projects.
Link: https://aasldpubs.onlinelibrary.wiley.com/doi/abs/10.1002/hep.30593