Bone is one of the most common sites of early metastasis of cancer and is also a hotbed for tumor cells. Why do tumor cells go after hard bones first? The latest study by teams from Prof. FAN Shunwu and Prof. LIN Xianfeng’s Lab from Sir Run Run Shaw Hospital (SRRSH), affiliated with Zhejiang University School of Medicine, found that they are directed at osteoclasts, the only cells in the human body that can secrete acid and dissolve bone. By feeding them closely, tumor cells ripen osteoclast-precursor cells and fuse them into larger and more destructive osteoclast named tumasteoclast, which acts like a crazy ice-breaker to carve out habitable zones on hard bone.
Based on this finding, the research team proposes a novel circuit breaker strategy for bone metastasis: nano-scale anti-osteoclast landmines are placed on the bone surface. Once tumor cells are coupled with tumasteoclasts, the landmines will be triggered immediately, physically killing the tumor cells and tumasteoclasts, thus stifling bone metastasis in the cradle. On March 18, 2024, Targeting initial tumour-osteoclast spatiotemporal interaction to prevent bone metastasis was published in Nature Nanotechnology.
Why do stones become hotbeds?
Metastasis is the most dangerous part of cancer development. Some tumor cells will enter the blood, circulate throughout the body, and then settle down away from the primary site. Clinical statistics show that bone is one of the earliest sites of metastasis. Tumor cells often use bone as a springboard to spread to other organs such as the liver, lungs and the brain.
Puzzlingly, hard bones do not seem to be habitable. The large amount of calcium salt in bone tissue constitutes a strong iron bastion, which will greatly hinder the proliferation and growth of tumor cells. But in fact, bone metastases develop faster than metastases in other sites due to the abundance of cell growth factors in the bone marrow. This makes Prof. Fan very confused: since softening the bone matrix is a prerequisite for metastasis, how do tumor cells bite the hard bone? To spread cancer to the bone is like planting flowers on a stone. Prof. Fan believes that if we find the answer, it is possible to find a way to prevent bone metastasis early.
While observing histological sections of the bone metastasis, PhD student GU Chenhui noticed that the metastasis was surrounded by many osteoclasts. While this is a classic phenomenon that has been observed before, the team believes that previous studies have not fully explained the reason for such close proximity and high density. What are the tumor cells and osteoclasts doing when they are so close? This key question led them to a discovery.
Tumor cells feed tumasteoclast closely
Osteoclasts are the only cells in the human body with the function of acid secretion and bone lysis. They usually stay in the bone tissue in the reserve state. Under certain conditions, they can be stimulated and integrated into giant mature osteoclasts. A small number of mature osteoclasts are responsible for absorbing bone, and then osteoblasts secrete calcium salts to regenerate bone, thereby maintaining a certain level of bone renewal. This process is similar to repairing a broken pavement, where osteoclasts are responsible for removing the broken pavement and osteoblasts are responsible for repaving the pavement, Gu said. Clinically, the overactivity of osteoclasts is one of the direct causes of osteoporosis.
The research team first discovered the crime tool of tumor cells. Under the scanning electron microscope, Gu found that the edges of the osteoclasts were very rough, which turned out to be many micron-scale vesicles with peduncles. The research team determined that this was the new organelle, the migrasome, recently discovered by Prof. LI Yu of Tsinghua University. The vesicles we observed are very similar to the migrasomes in terms of formation, shape, and size, Gu said.
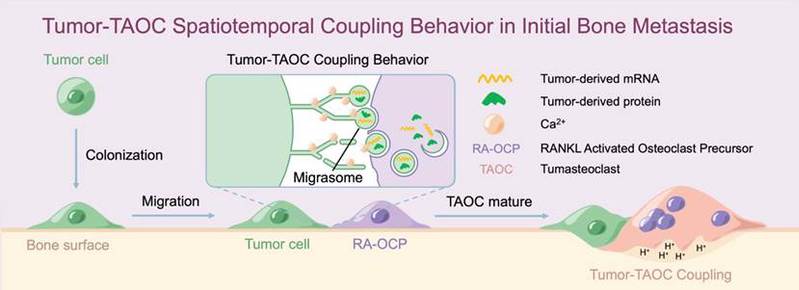
It turns out that as soon as tumor cells land on the surface of bone, they “make up to” osteoclasts. Tumor cells transfer RNA, protein and other cytoplasmic components to osteoclast-precursor cells through the migrasomes, and ripen them in large quantities. Opening this recipe presented by tumor cells, the researchers found mRNA associated with non-classical osteoclast differentiation transcription complex. Tumor cell-derived mRNA allows osteoclast precursor cells to differentiate into osteoclasts under abnormal conditions, said TIAN Hongsen, a doctoral student. Tumor cells are metabolically active, and their special RNAs and proteins can speed up the metabolism of normal cells, Dr. CHEN Pengfei explained. It makes the osteoclast precursor cells fuse into a bruiser.
The research team believes that this tumor-coupled osteoclast in tumor metastasis is different from previous osteoclasts in terms of induction mode, mediator and transcription pattern, and thus represents a new subtype of osteoclast. We named it tumasteoclast (TAOC), said Prof. LIN Xianfeng. After ripening and fusion, tumasteoclast, a frenzied acidic machine, helps the tumor cells absorb bone and remove obstacles.
Set up landmines to stop the metastasis
In clinical practice, bone metastasis of tumor cells is very common. Prof. FAN Shunwu, who has been a doctor for more than 40 years, has contacted many patients who suffer from joint pain and mobility difficulties, which may be related to bone metastasis. According to reports, there are still limitations in the current diagnosis and treatment of bone metastases: the lesions generally have to reach the millimeter-scale before they can be detected by imaging methods such as X-ray and CT. We have to find a way to 'kill' tumor cells when they induce tumasteoclasts at an early stage. Prof. Fan said that only by achieving cell-level tumor killing, it is possible to stifle bone metastasis in the cradle and achieve the effect of prevention.
Based on the new findings, the research team proposed that the coupling behavior of tumor cells and tumasteoclasts should be targeted. Tumor cells are very cunning. In conventional tumor cell targets, specific molecules are often used as targets, but tumor cells may take 'disguise' and other means to evade recognition. Prof. Lin introduced that the team proposed the idea of behavior targeting to tumor cells by recognition of tumasteoclasts.
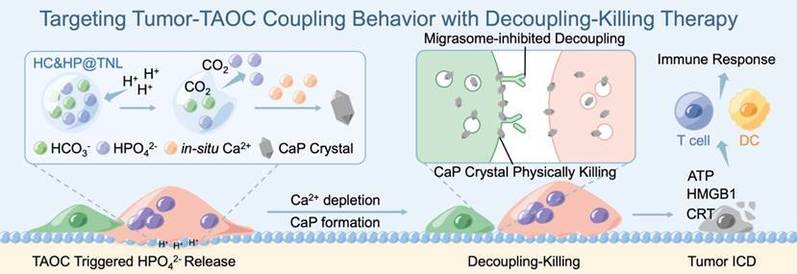
The team has previously developed liposomes that specifically release sodium bicarbonate in the acidic sealing zone of osteoclasts for preventing osteoporosis, and the related research is published in Journal of the American Chemical Society. Inspired by this strategy, they developed a new type of anti-osteoclast landmine. Dr. Chen introduced that there are two substances in this nano-scale liposome landmine: one is sodium bicarbonate, which can react with acid secreted by osteoclasts to produce gas, thus detonating the landmine; The other is sodium hydrogen phosphate. After the mine is detonated, hydrogen phosphate ions will form sharp calcium-phosphorus crystals with calcium ions in situ, which stab into the cell membrane of tumor cells and cause physical damage.
The team also embedded tetracycline molecules on the surface of the anti-osteoclast landmines, which allowed them to be directed to the bone surface. Once tumor cells and tumasteoclasts are coupled, acidic substances from tumasteclast will immediately detonate landmines. This is when the tumor cells step on the 'land mine', Prof. Lin introduced, Then formation of a large number of calcium-phosphorus crystals can effectively kill tumor cells. Because sodium bicarbonate and sodium hydrogen phosphate are existing substances in the body, they have biological safety. This protocol has been validated in a mouse model of bone metastases. The team said that the effect with the nanoliposomes was surprising. Animal experiments have shown that the combination of the landmine and anti-PD-1 antibody can inhibit bone metastases by about 90%.

At present, the team has completed the verification of the effect of a variety of animal bone metastasis models, simultaneously submitted the invention patent, and started to translate the research into clinical practice. The research team believes that the success of this study is due to the interdisciplinary advantages of Zhejiang University and the free exploration atmosphere of SRRSH. The orthopaedic team at SRRSH has long been engaged in the mechanism of musculoskeletal diseases and the treatment strategy of biomaterials, and actively explored the possibility of clinical translation. The innovation of the study was unanimously recognized by the reviewers, and was invited by the editor to share Behind the Paper: Tumor-Osteoclast Spatiotemporal Coupling: A Cell Behavior Target for Bone Metastasis Prevention.
More information: Prof. LIN Xianfeng and Prof. FAN Shunwu of SRRSH, are the co-corresponding authors of this article. Doctoral student GU Chenhui, Dr. CHEN Pengfei, and doctoral student TIAN Hongsen from SRRSH are the co-first authors of this paper.
Link to the article: https://doi.org/10.1038/s41565-024-01613-5
Source: Sir Run Run Shaw Hospital, Zhejiang University School of Medicine