Voltage-gated ion channels (VGICs) are involved in electrical signal transduction and play essential roles in life activities. How membrane potential drives conformational change at the voltage-sensing domain (S1-S4, VSD) and regulates channel gating is a central theme for voltage-gated ion channels. To elucidate voltage-gating mechanisms, one needs to capture VSD structures in both the activated state and resting state.
In recent years, the research team led by Prof. GUO Jiangtao from Zhejiang University School of Medicine has conducted systematic research into the voltage gating mechanism of TPC1. The latest research results were published in the journal PNAS on November 30, and entitled “Voltage-gating and cytosolic Ca2+ activation mechanisms of Arabidopsis two-pore channel AtTPC1”.
AtTPC1 belongs to the VGIC superfamily. It is localized in the vacuolar membrane and is responsible for generating slow vacuolar (SV) current. In addition to being activated by membrane depolarization, AtTPC1 is also doubly regulated by Ca2+. It is activated by cytosolic Ca2+ binding at the EF-hand domain but inhibited by vacuolar Ca2+ binding at VSDII. To obtain the structure of AtTPC1 in open-state with the presence of Ca2+, based on the previous study, researchers used the mutant AtTPC1ΔCai with three Ca2+-coordinating residues on VSDII (D240A/D454A/E528A), which can mitigate the vacuolar Ca2+ inhibition.
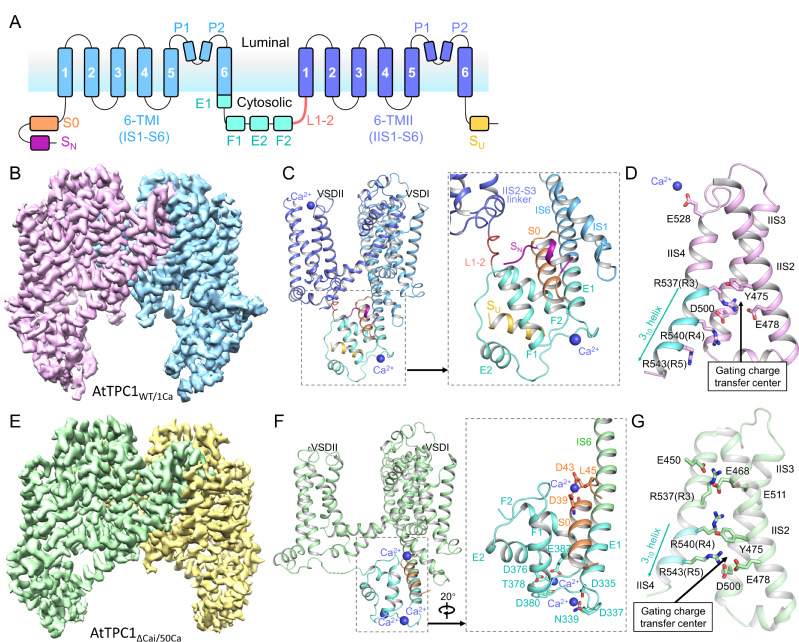
Cryo-EM structures of AtTPC1WT/1Ca and AtTPC1ΔCai/50Ca
Researchers determined the 2.8 to 3.3 Å cryo-electron microscopy (cryo-EM) structures of AtTPC1 in two conformations, one in closed conformation with unbound EF-hand domain and resting VSDII, and the other in a partially open conformation with Ca2+-bound EF-hand domain and activated VSDII. Structural comparison between the two different conformations allows us to elucidate the structural basis of gating mechanisms in AtTPC1.
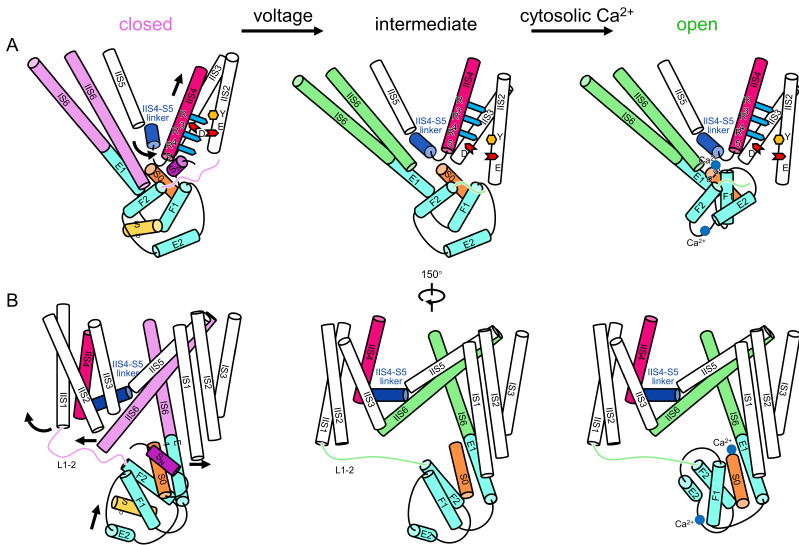
The voltage gating, cytosolic Ca2+ activation, and their coupling mechanism in AtTPC1
Upon membrane depolarization, the global movement of VSDII is initiated by the vertical translation of IIS4 with gating charge residues passing through gating charge transfer center. The activation of VSDII not only causes IIS6 helix pair to dilate further away from the central axis of the channel pore, but also pulls the EF-hand domain through L1-2, allowing the Ca2+ binding sites at S0 and EF hand 2 to be exposed. The binding of Ca2+, together with dragging by VSDII, induces the translation and rotation of E1 away from its original position. Since E1 and IS6 form a single helix, the motion of E1 directly causes an outward movement of IS6. With the enlarged pore along the directions of IS6 and IIS6 pairs, the channel opens.
"Structural comparison between the two different conformations allows us to elucidate the structural mechanisms of voltage gating, cytosolic Ca2+ activation, and their coupling in AtTPC1." said Prof. GUO, "This study provides structural insight into the general voltage-gating mechanism among voltage-gated ion channels, and will guide the development of drugs targeting two-pore channels and other voltage-gated ion channels."
Source: GUO Jiangtao'Lab