SARS-CoV-2 utilizes its spike protein to recognize host angiotensin-converting enzyme II (ACE2) receptor, which induces subsequent S1/S2 detachment of spike for forming fusion machinery to invade host cells. However, only small structural changes of spike occur upon ACE2 binding, which raises questions whether these small conformational changes are enough to trigger the detachment of tightly associated S1/S2 subunits. If not, what additional factors are required?
The research team led by Dr. CHEN Wei from the School of Basic Medical Sciences published an article entitled “Mechanical activation of spike fosters SARS-CoV-2 viral infection” online on August 31 in Cell Research. This research demonstrates that the mechanical force can enhance spike/ACE2 interaction and accelerate the S1/S2 detachment, thus facilitating viral invasion.
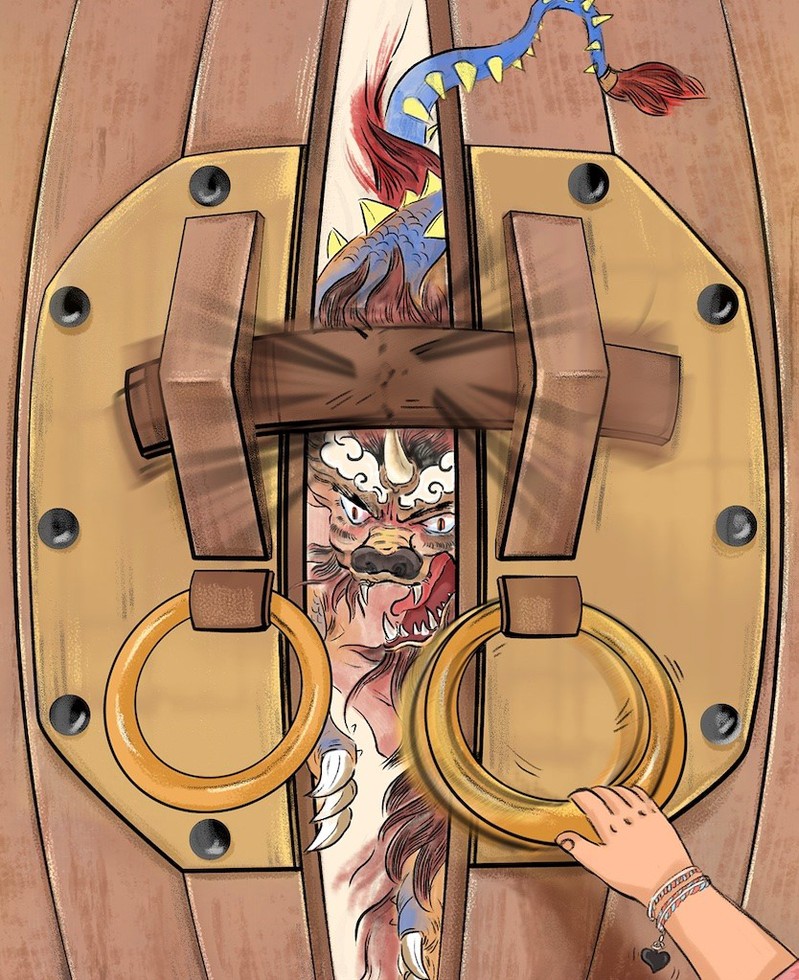
A kid’s hand (ACE2) pulls the door knocker (SARS2-RBD), helping to release a monster (SARS2-genome) by facilitating to break up the door threshold (S1/S2 lock).
Once a virion attaches to the host cells, the pulling force on the spike/ACE2 bonds at the contact zone edge is the roughly ranging from 0 to 30 pN according to the theoretical analysis of this research. This pulling force would enhance the invasion of SARS-CoV-2 into host cells by mechanically strengthening its spike binding with host ACE2 receptors and by accelerating S1/S2 detachment to destabilize the pre-fusion spike trimer. Unexpectedly, this research finds that D614G mutation in spike (shared by Alpha, Beta, Delta, Gamma and Lambda strains) shows 3-time stronger force-dependent ACE2 binding and 35-time faster force-induced S1/S2 detachment, which provides a novel molecular mechanism to explain the high infectivity of D614G-related strains.
In addition, this study also identifies an anti-S1/S2 non-RBD-blocking antibody that is derived from convalescent COVID-19 patients and with potent neutralizing activity for virus infection can impede S1/S2 detachment by 3×106 times under force. Based on these findings, researchers propose an alternative non-RBD-blocking but S1/S2-binding neutralizing strategy: exploiting S1/S2-targeting mAbs to stabilize SARS2-S structure by locking SARS2-S conformation to prevent S1/S2 detachment and follow-up S2 fusion machinery formation. S1/S2-locking neutralizing strategy potentially can compensate or compliment receptor-blocking strategy no matter what other novel spike’s receptors are found.

The model for mechano-activation of SARS-CoV-2 spike and its inhibition by non-RBD-blocking, S1/S2-targeting, but neutralizing antibody
“Our study sheds light on mechano-chemistry of spike activation and on developing a non-RBD-blocking but S1/S2-locking therapeutic strategy to prevent viral invasion,” said CHEN Wei. “It is a successful case of interdisciplinary cooperation, and also a major breakthrough in the fields of biomechanics and mechanobiology, single molecule biophysics and virology.”
Source:CHEN Wei's Lab