In a research article published on April 12, 2019 in Science titled “Structure and Dynamics of the Active Human Parathyroid Hormone Receptor-1” (DOI: 10.1126/science.aav7942), scientists at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences led by Huaqiang Eric Xu and Ming-Wei Wang, Zhejiang University School of Basic Medical Sciences led by Yan Zhang and University of Pittsburgh School of Medicine (USA) led by Jean-Pierre Vilardaga, revealed a high-resolution cryo-electron microscopy (cryo-EM) structure (3.0Å) of the human parathyroid hormone type 1 receptor (PTH1R) bound to a long-acting parathyroid hormone (PTH) analog and the stimulatory G protein. It provides valuable insights into structural basis and dynamics of PTH binding and long-term activation of the receptor, thereby laying a solid foundation for discovering novel therapeutics against osteoporosis and other diseases.
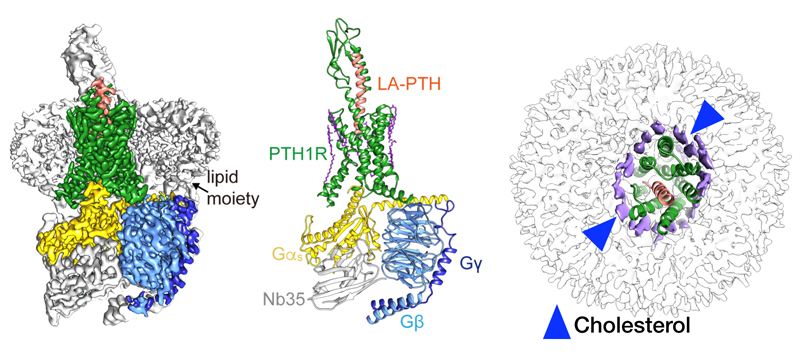 PTH is a classic endocrine hormone, which had been identified over 80 years ago, and plays critical and distinct roles in skeletal development, calcium homeostasis, and bone turnover. The functions of PTH are mediated primarily through binding and activation of PTH1R, a member of the class B G protein-coupled receptor (GPCR) subfamily. As a well-recognized drug target for osteoporosis, PTH1R is highly expressed in bone and kidney cells where it exerts regulatory action in calcium and phosphorus metabolism. Analogs of PTH (such as Teriparatide acetate) are presently used in the clinic to treat osteoporosis.
PTH is a classic endocrine hormone, which had been identified over 80 years ago, and plays critical and distinct roles in skeletal development, calcium homeostasis, and bone turnover. The functions of PTH are mediated primarily through binding and activation of PTH1R, a member of the class B G protein-coupled receptor (GPCR) subfamily. As a well-recognized drug target for osteoporosis, PTH1R is highly expressed in bone and kidney cells where it exerts regulatory action in calcium and phosphorus metabolism. Analogs of PTH (such as Teriparatide acetate) are presently used in the clinic to treat osteoporosis.
Scientists and graduate students of the abovementioned four research groups worked diligently and cooperated closely with each other and eventually determined the three-dimensional structure of a long-acting ligand (LA-PTH) bound to PTH1R-Gs complex. This structure demonstrates detailed interaction between LA-PTH-bound PTH1R extracellular domain (ECD) and transmembrane domain (TMD) and offers a comprehensive understanding of how PTH1R interacts with a peptide agonist and couples to Gs. It is the first full-length PTH1R structure in an active state and the first three-dimensional GPCR structure under the long-term activation condition. It also reveals the intracellular cAMP signaling mechanism of PTH1R in a prolonged active state. Due to the high resolution (3.0Å) of this complex, the scientists unexpectedly discovered the extensively ordered lipid distribution around the TMD which may increase conformational stability of the receptor.
In previous studies, it has been speculated that endogenous ligand binds and activates class B GPCR through a “two-step” model: the carboxyl terminal of the ligand first binds the extracellular domain of the receptor, and subsequently its amino terminus inserts into the hydrophobic pocket of the transmembrane domain. However, how ligand dissociates from receptor remains unknown. Parathyroid hormone can not only activate its receptor quickly, but also dissociate rapidly. Scientists prolonged its residence time on the receptor using a long-acting agonist, and subsequently captured the states of the ligand dissociating from the receptor by using delicate and meticulous three-dimensional classification during imaging analysis. The flexible extracellular domain of the receptor remains its inherent dynamic characteristics upon the ligand binding and exerts two effects on the helical ligand during the continuous movement: (1) it approaches the ligand to produce stress that prompts the ligand unwinding, and (2) it moves away from the ligand to weaken their interaction. The combination of these two effects leads to the dissociation initializing at the carboxyl end of the ligand. This study improved our understanding the molecular recognition mechanism of class B GPCR.Dr. Lihua Zhao and Shanshan Ma (Ph.D. candidate) from Shanghai Institute of Materia Medica, Dan-dan Shen from Zhejiang University and Ieva Sutkeviciute (Ph.D. candidate) from University of Pittsburgh are the four co-first authors of this paper. Contributing institutions also include Van Andel Research Institute (USA), Fudan University and Harvard Medical School (USA). The work was funded by Zhejiang Province Science Fund for Distinguished Young Scholars, Chinese Academy of Sciences, National Natural Science Foundation of China, Ministry of Science and Technology of China, National Health Commission of China, National Institutes of Health (NIH, USA), Shanghai Municipal Commission of Science and Technology, Fudan-SIMM Joint Research Fund and Novo Nordisk-CAS Research Fund and several career development grants from China and the United States (such as Youth Innovation Promotion Association of Chinese Academy of Sciences).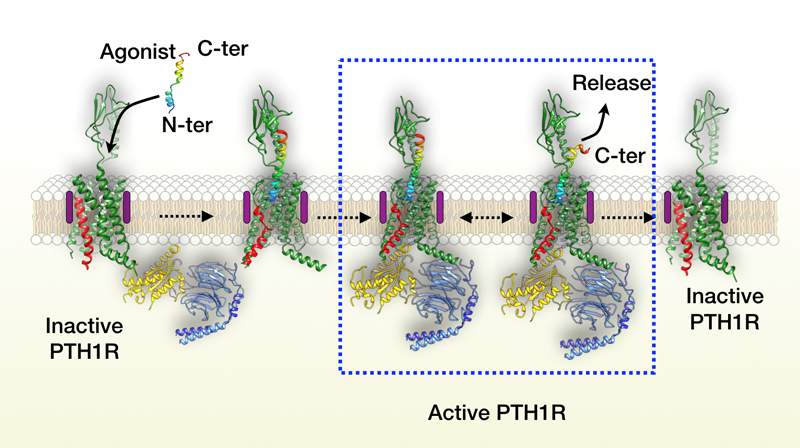
The cryo-EM data were collected at the Center of Cryo-Electron Microscopy, Zhejiang University.
The paper linker: https://science.sciencemag.org/content/364/6436/148.full